Innovative Peptide-Oligo Conjugate Manufacturing Solutions
July 18, 2025
Peptide Drug Conjugates (PDCs) have shown remarkable progress in targeted delivery and enhanced therapeutic efficacy. However, challenges remain in manufacturing—particularly in scaling up, optimizing yields, balancing cost-efficiency, and ensuring quality through robust analytical methods.
Our trusted partner provides a comprehensive, one-stop solution for peptide synthesis, oligonucleotide synthesis, and conjugation (e.g., PPMO), enabling seamless and efficient project execution. Their expertise in continuous purification and impurity analysis significantly improves both yield and quality control throughout the process.
In our latest white paper, you’ll find:
- Practical solutions to purification and analytical challenges in PDC manufacturing
- Insights and experiences in synthesizing complex peptides, oligonucleotides, and conjugates
Conjugation examples
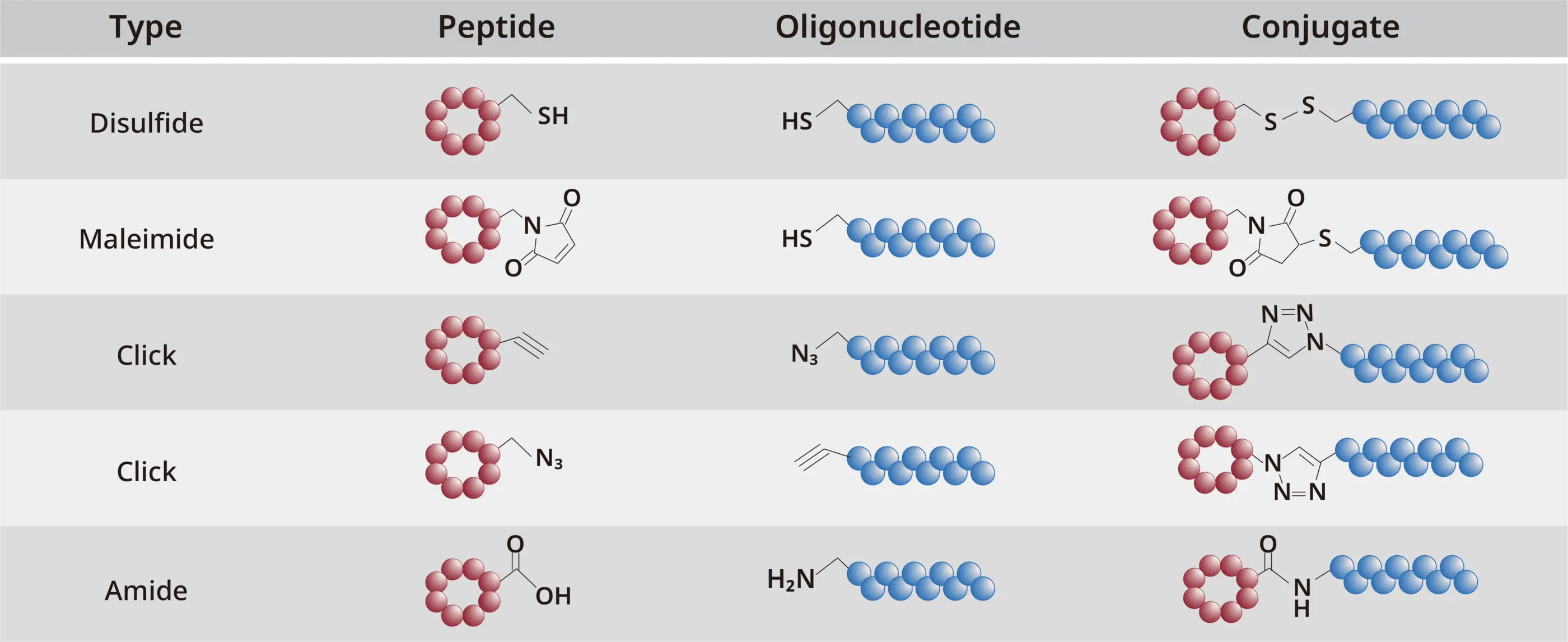
We support efficient PDC development through timely, precise technical communication and tailored proposals grounded in deep industry expertise. These capabilities help streamline processes, reduce in-process testing, and shorten lead times—ensuring consistent delivery of high-quality outcomes.
Discover how our CDMO services can fast-track your peptide, oligo, and conjugate development!
To download the full paper, please enter your information below.












